Accuracy and reproducibility
Cell research is critical across multiple fields, where precise calculation of cell count and viability is a key of step. According to GB/T42076.1-2022, both the hemocytometer method and image analysis method are current national standard methods for cell counting. The efficiency of cell counting (usually measured by the time taken per sample), accuracy (typically benchmarked against manual counting), and reproducibility (commonly evaluated by the coefficient of variation, CV, from multiple measurements of the same sample) are essential parameters for cell counting devices. Newtonoptic cell counter utilizes automated image acquisition combined with proprietary algorithms to deliver test results such as cell concentration and viability within one second, providing highly reproducible data. This enables users to rapidly obtain accurate experimental data.
Accuracy
Target:
Multiple cell samples with known concentrations were obtained by performing serial dilutions of a cell sample with a known concentration. These samples were measured using the cell counter to validate the accuracy of the cell counting results and the linearity of dilution within this concentration range.
Experimental protocol:
1、After harvesting and centrifuging the HEK293 cells, the cell pellet was resuspended in PBS. Manual counting determined the concentration of the cell suspension to be 8.606 × 10⁶ cells/mL. The sample was labeled as Cell1;
2、The Cell1 suspension was serially diluted with PBS at 2-fold, 4-fold, 8-fold, and 16-fold dilutions to obtain Cell2, Cell3, Cell4, and Cell5, respectively.;
3、Samples Cell1 through Cell5 were measured using the Newtonoptic Tunin cell counter to determine cell concentration and viability.
Result:
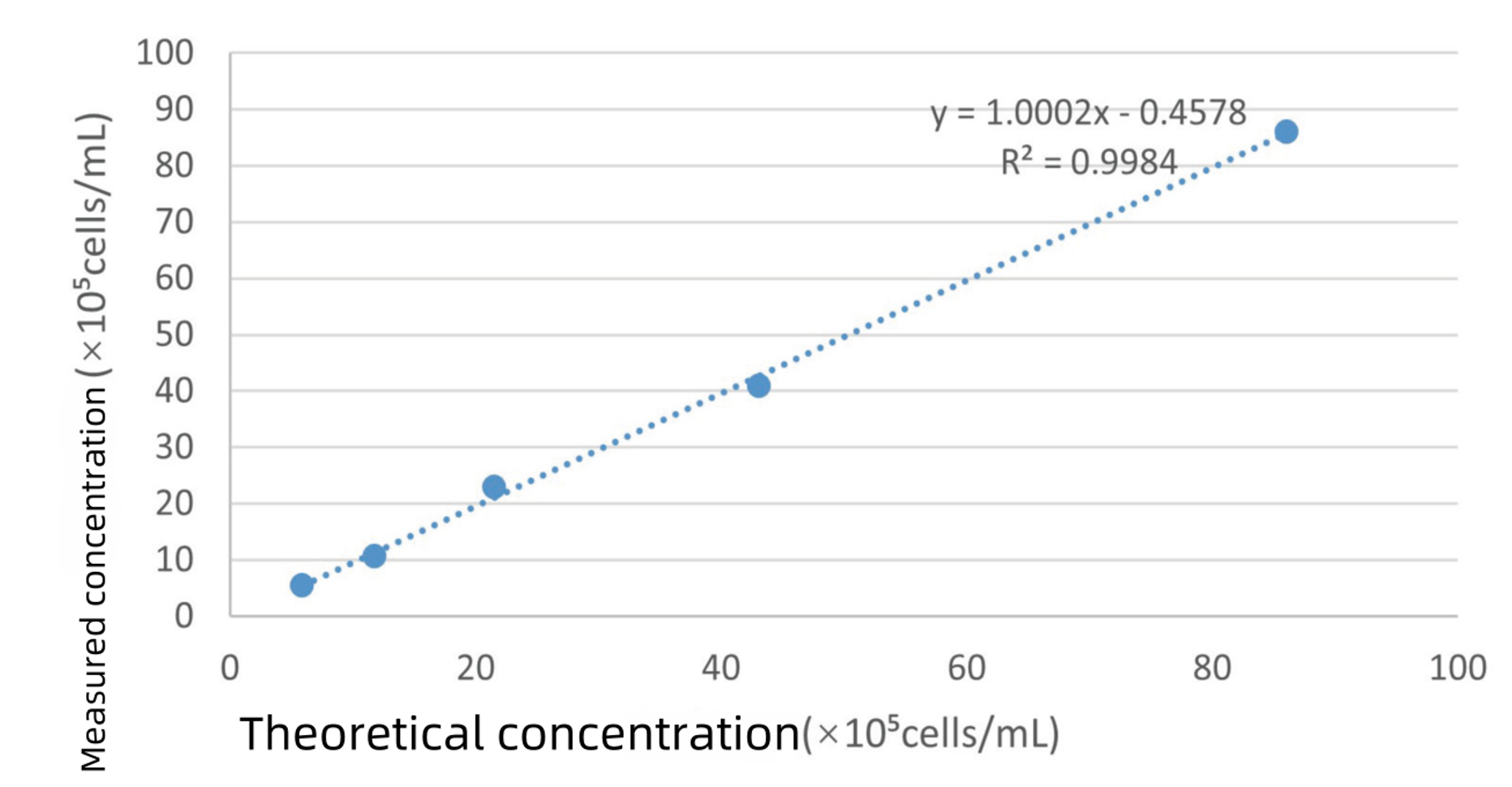
Correlation/Fitting analysis between the measured and theoretical cell concentrations
Conclusion:
A comparison between the theoretical and measured concentrations demonstrated that, within the cell concentration range of 5 × 10⁵ to 8 × 10⁶ cells/mL, the Newtonoptic cell counter provided accurate results with good dilution linearity. The fitting of the counting results yielded an R² value greater than 0.99.
Reproducibility
Target:
The reproducibility of the cell counter was validated by performing multiple measurements on HEK293 cell samples at different concentrations.
Experimental scheme:
1、Three representative concentrations of HEK293 cells — high, medium, and low — were prepared, and the samples were labeled as Cell1, Cell2, and Cell3, respectively;
2、The three cell concentrations were analyzed using the Newtonoptic Tunin cell counter. Each sample was measured six times to evaluate the reproducibility of the counting results;
3、Manual counting was performed using a hemocytometer and compared with the instrument analysis results. The results are presented below:
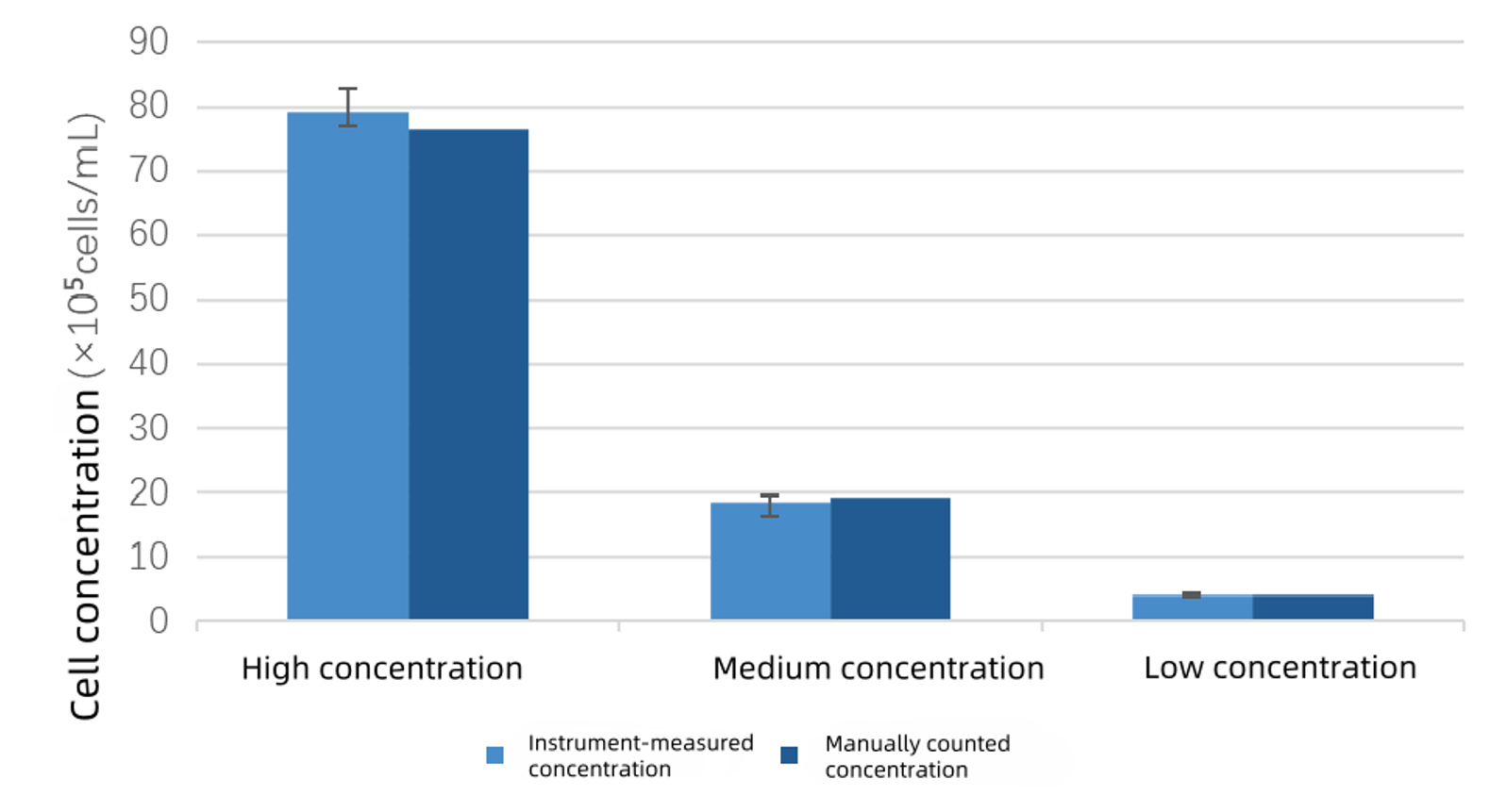
Comparison between instrument-measured concentration and manual counting concentration
![]()
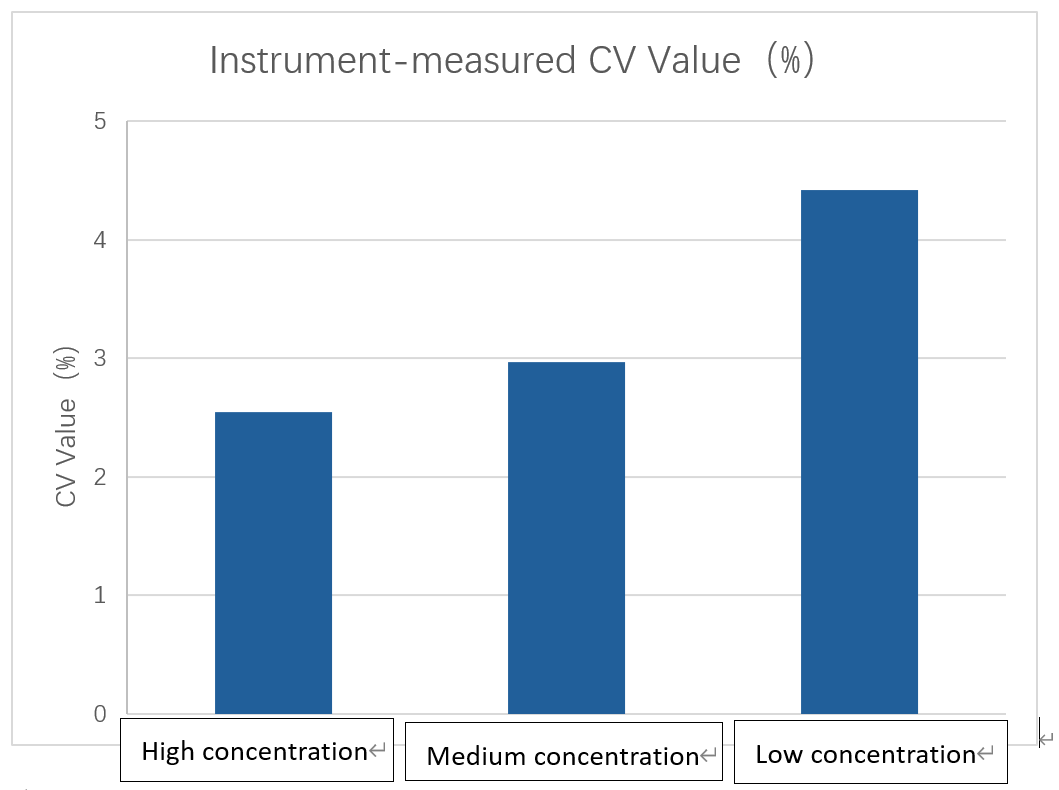
CV values of concentration measurements for high, medium, and low cell samples
Conclusion:
The counting results from the Newtonoptic cell counter were close to those of manual counting. The Newtonoptic cell counter demonstrated excellent reproducibility, with CV values all within 5%.

National Advisory Service Hotline
Sales consultation: +86 15322248165(Whatsapp/Wechat)
E-mail:global@newtonoptic.com
R & D Center: Room 301, Floor 1, Building 1, No.38 Gaopu Road, Tianhe District, Guangzhou City, Guangdong Province

Follow Wechat Official Account
©2024 Guangzhou Newtonoptic Research Institute Co., Ltd. All rights reserved